Understanding the Biological Phosphorus Removal Process:
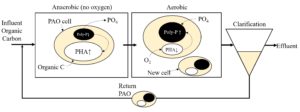
Schematic of the BPR Process.
Biological phosphorus removal (BPR) is a well-established method that selects for PAOs, who can store up to 15 – 20% of their dry biomass in phosphorus. The phosphorus is stored as a polymer called polyphosphate (poly-P), which can be conceptualized as a battery for the PAOs. A typical BPR configuration is an anaerobic zone (absence of oxygen) followed by an aerobic zone. Under anaerobic conditions, the PAOs are forced to use stored energy reserves and thus metabolize the intracellular poly-P for energy supply. The energy deliberated from poly-P cleavage is used to take up organic carbon from the wastewater, which gets stored internally by PAOs as a carbon polymer, called polyhydroxyalkanoates (PHA). Under aerobic condition, the PAOs can oxidize the PHA to produce energy, which is then used to grow new cells and resynthesize the poly-P storage. For polyphosphate synthesis, the PAOs must take up the phosphate from the liquid and incorporate it intracellularly, by which a net removal of phosphate from the liquid is achieved.
Proposed Sensor Concept and Design:
To simplify and advance BPR, phosphate (PO43-) sensor development remains an area of active research, with none capable of accurate PO43- quantification in complex wastewaters. Poly-P consists of Mg2+, K+ and PO43- in a 1/3: 1/3: 1 molar ratio. Thus, during synthesis or breakdown of the poly-P, for every mole of PO43- ions transported, 1/3 mole of Mg2+ and 1/3 mole of K+ ions (secondary metabolites) are co-transported into and out of the cells, respectively. Using the known relationship between the secondary metabolites and PO43-, we propose a multi-sensor design that combines the commercially available K+ and Mg2+ probes to indirectly quantify phosphate concentrations. The molar ratio of PO43- and secondary metabolites during release and uptake of phosphorus has been shown to vary due to the complexities of wastewater. Also, the complex makeup of wastewater is expected to lead to interferences in K+ and Mg2+ probes. Therefore, it is important to note that a simple physics model (measuring only the secondary metabolites and calculating phosphorous) is insufficient for this application. Instead, we will develop a machine learning algorithm trained with data obtained across multiple sensor signals (a multi-sensor array) in a stable bioreactor to quantify phosphate in real time. In other words, the multi-sensor technology does not rely on a fixed molar ratio of K+/P and Mg2+/P. The neural network will incorporate the underlying factors that drive the variability in ratios.
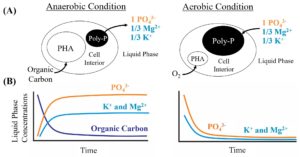
(a) Schematic of the PAO metabolism. (b) Release and uptake of P, Mg and K in coupled ratios.
All images © 2018 Winkler Lab
